As a teenager, Hugh Herr got lost during a blizzard on a mountain. He surived the ordeal but was gravely injured. “Both of my legs were amputated just below the knee due to tissue damage from frostbite,” he explained in a 2020 talk. As he adjusted to his new body, he said, “I began to imagine a world with such advanced technology that disability is no more.”
Herr has done more than just imagine this world—he is helping to create it. He returned to mountain climbing, using legs and feet he designed himself. Today, Herr is an engineer at MIT in Massachusetts who develops devices that merge biology with electronics and mechanics. Some of these devices interface with the human nervous system or brain, making it possible for people to control mechanical limbs with thought alone. A mind-controlled robotic limb is called a neuroprosthesis.
Thanks to the efforts of people such as Herr, this incredible technology is moving from the world of science fction into reality.
Hijacking Nerve Signals
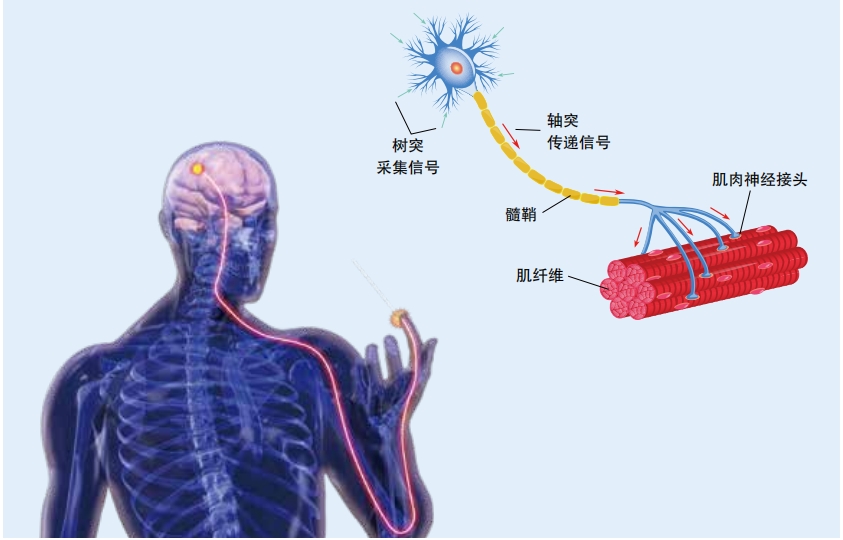
“When you think you want to move—you want to rotate your wrist, or you want to move your fingers—the signal travels from your brain down your spinal cord, and then out the peripheral nerves from the spinal cord into your arm,” said Tyler Hayes, the CEO of Atom Limbs, a company that makes neuroprosthetics.
“Even when someone has lost or damaged a limb,” Hayes continued, “those nerves are still there and they’re still firing into muscles, it’s just that there’s no real hand left to move. So we listen to the electrical field emanating from your arm, from your muscles, and just tap into that exact signal that your body is sending. ”
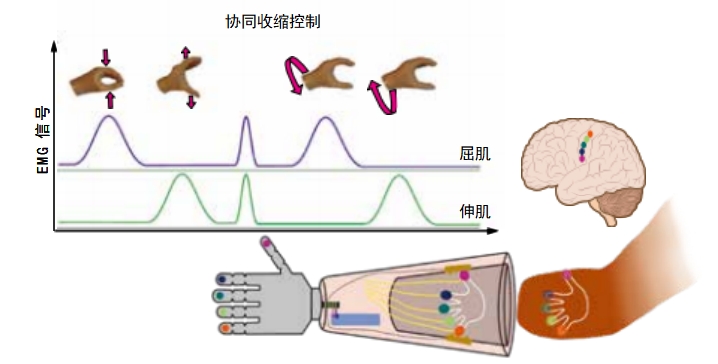
Many neuroprosthetics take advantage of the system of nerves traveling from the brain throughout the body. Some robot arms and legs currently available for everyday use read muscle twitches in remaining parts of the body. This is called a myoelectric prosthesis. For example, a person with an amputated arm might twitch one muscle in her shoulder to open a robotic hand, then twitch a different muscle to close it. Often, these types of limbs can only perform a few very simple motions. Around half of the people who receive these devices stop using them out of frustration. But more advanced versions of myoelectric prosthetics are adding more motions and becoming easier to use.
The limbs that Atom Limbs makes use advanced sensors and machine learning—where computers train themselves to become more accurate—to interpret electrical signals traveling down the nerves from a person’s brain.They basically hijack the body’s own signaling system in order to move and manipulate a prosthetic limb. Its Atom Touch is the first prosthetic arm that offers control of individual fingers in the hand. Users wear a cuff over the part of their arm that is still there. This cuff contains electrodes that sense signals coming from the nerves in the remaining muscles.
Paul Carter was born without lower arms and legs, but he does have muscles in his upper limbs that have working nerves. In 2024, he tried a computer simulation of an Atom Limbs prosthetic. He called it a “mind-boggling experience. ” He wore the cuff of sensors and assigned his upper arm muscles to hand, wrist, and elbow movements. Then he practiced moving a virtual arm shown on a computer screen. “The notion of learning how to control a part of the body I don’t have is almost impossible to describe,” he said.
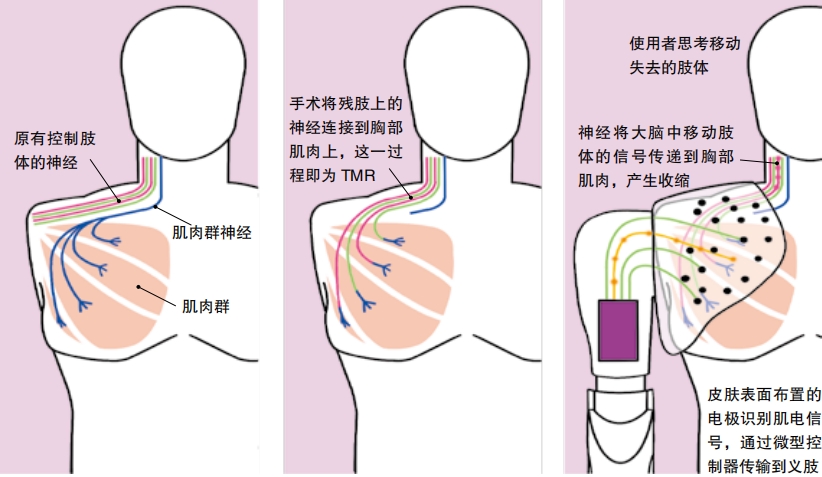
Claudia Mitchell lost her arm in a 2004 motorcycle accident and has helped to test a myoelectric arm and hand developed at the Cleveland Clinic in Ohio. She went through a procedure called targeted muscle reinnervation (TMR) . During surgery, doctors moved nerves that used to lead to her arm and hand into her chest muscles. When she wears the arm, electrodes sit just over these muscles. So thinking about moving her hand causes these muscles to twitch a certain way, which alerts the electrodes that the hand should move a certain way. She’s not thinking about moving her chest muscles, though. She only thinks about moving her hand. “Even though your hand’s not there, the nerves that used to connect to your hand are connected to muscle and skin, and so your brain doesn’t really know that your hand’s not there at that point,” says Paul Marasco. As a doctor and researcher at Cleveland Clinic’s Laboratory for Bionic Integration, he led the development of this device.
ANew Kind ofAmputation
Amy Pietrafitta’s left leg had to be amputated below the knee in 2018 after a bad burn. A few years later, this para- athlete from Massachusetts was at Hugh Herr’s lab, walking normally again. She was testing an advanced neuroprosthetic device as part of a research study. “I didn’t feel like my leg had been amputated,” she told CNN. “It was the happiest moment in my life. ” She had to stay in the lab holding onto handrails during the test, but she said, “I kept wanting to take my hands off, to get out and start moving. ”
The research study Pietrafitta took part in was published in July 2024. A control group of seven participants had received a typical, old-fashioned below-the-knee leg amputation. Pietrafitta and six others also had below-the-knee amputations, but they had received a special surgical intervention called an agonist-antagonist myoneural interface or AMI.
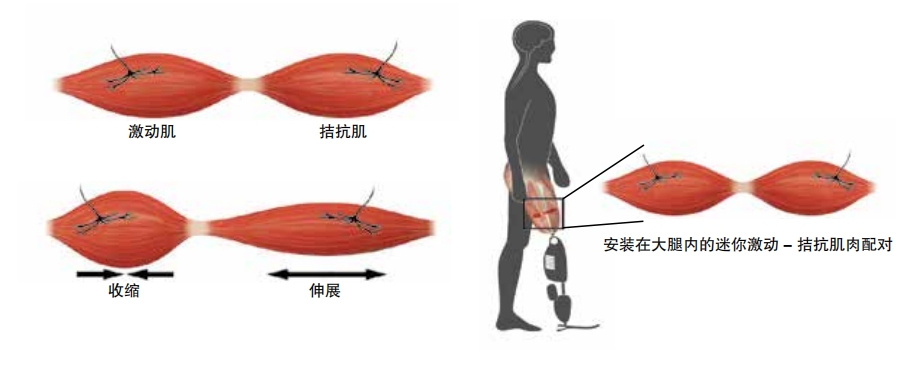
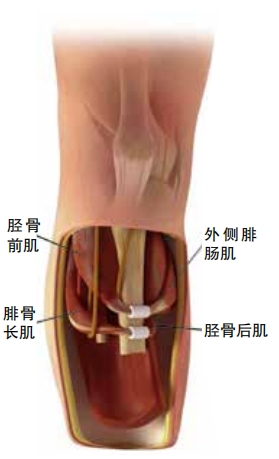
The goal of an AMI is to maintain nerve signaling between the brain and a missing limb even when most of the limb is gone. To understand how this works, it’s important to understand a bit about how muscles work. Skeletal muscles operate in pairs, called agonists and antagonists. For example, the hamstring in the back of the leg is an agonist. It pairs with the quadriceps in the front of the leg, an antagonist. Whenever an agonist squeezes tight, the antagonist stretches out. This stretching sends feedback along the nerves to the brain. This helps the brain understand where the muscles are and what they are doing. Sensing where the body is in space is called proprioception.
To build an AMI, a surgeon constructs mini versions of muscle pairs within the remaining part of the limb. So if the leg is getting amputated above the knee, mini versions of muscles from the lower leg will have to be rebuilt inside the upper leg. The surgeon can use pieces of the original muscles. Or thesurgeon may move small amounts of muscle tissue from elsewhere in the body or use other techniques to rebuild the pairings. The surgeon also makes sure that these muscle pairs connect to the correct nerves.
The end result is that all of the nerves that used to lead to a full set of muscles now lead to tiny sets of muscle tissue. Even though the full limb is no longer there, the brain can communicate normally with the bits of muscle. That means the brain can still tell the muscles when to contract or stretch. And a neuroprosthetic can pick up these signals to tell a robotic limb how to move.
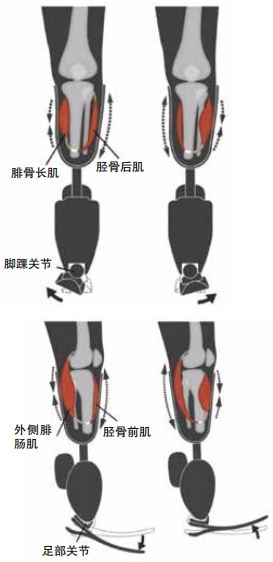
The most interesting thing about an AMI, though, is that it keeps the sense of proprioception intact. When a body part is amputated without an AMI, this sense can go haywire, leading to itching or even pain in a limb that isn’t there. This is called phantom limb syndrome. But the AMI allows the brain to understand where the limb is based on feedback from the muscle pairs. An AMI works best when it’s done during the original amputation but can sometimes still succeed when done within a few years afterward.
In the study, Herr’s team fitted each amputee with a battery-powered prosthetic leg and a small electronic controller. The participants with an AMI also had electrodes on the skin at the amputation site. These electrodes picked up nerve signals traveling to the muscle pairings and translated these into instructions for moving the prosthetic leg. Past experiments had shown that nerve signals could control a bionic limb during specific phases of movement, such as the swing phase of walking. This experiment marked the first time that the mind remained in control during all movements. And it was a huge success. The participants with AMIs could walk up slopes and over obstacles. On flat ground, they walked at a speed equivalent to people without any amputation—41 percent faster than the control group who hadn’t received an AMI.
The electrodes did not send any signals from the prosthetic back to the brain. However, the AMI meant that the brain could still form a sense of where the muscles were in space, likely from the way the residual muscles responded. “So when the amputee thinks, they feel the limb moving naturally,” Hugh Herr told the BBC. “It’s as if they are able to feel their phantom limb and its movement,” he said.
Pietrafitta and others like her will have to wait to use this amazing prosthetic in their daily lives. It is an expensive prototype that has not yet been approved or made available for everyday use.
Regaining Sensation
Most prosthetic devices lack touch sensations. That’s a big problem because touch is essential to most things people want to do with their prosthetics. Picking up something with an intact hand involves not just moving the arm and fingers but feeling the item so you know when to grip and how hard to hold on.Walking involves not just seeing where your legs are going but feeling the pressure of the ground underfoot. It can be very difficult and frustrating to use a prosthetic limb that has zero sensation.
Touch is also important for our well-being. “Touch is about connection,” says researcher Dustin Tyler. “It’s connection to the world. It’s about connection to others. And it’s connection to yourself, right? We never experience not having touch—it’s the largest sensory ‘organ’ on our body.” Tyler is a biomedical engineer at Case Western Reserve University and the Cleveland VA Medical Center in Ohio.
The Atom Touch device offers basic touch sensation, also called haptic feedback. When the fingers of the device contact something, the cuff buzzes. Some video game controllers offer a similar type of feedback, with the controller buzzing after a big hit or other event in the game. This is a good start, but it’s a far cry from the full range of sensations that a real hand can feel.
More advanced prosthetics that aren’t yet available for daily use have tested ways of providing more detailed haptic feedback. Brandon Prestwood worked with Tyler to help test one such device. Prestwood had lost his left hand in an industrial accident. In 2016, he volunteered to have electrodes implanted into the remaining part of his arm, over the nerves . These electrodes picked up signals from his brain and transmitted those to a computer that controls a prosthetic hand. So he could move the hand just by thinking about it. The electrodes also helped provide a sense of touch. Plastic caps over the fingers on the prosthetic hand contained sensors that track touch. When these sensors detected that the prosthetic was touching something, the electrode implant would activate his nerves with tiny jolts of electricity.
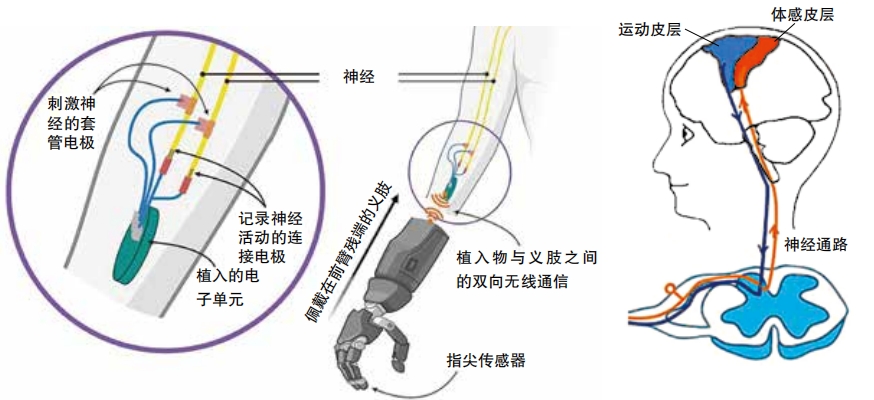
When Tyler first tried this procedure with a human volunteer in 2012, he had no idea what might happen. Thankfully, it wasn’t. In a segment aired on 60 Minutes in 2023,Prestwood described what it was like to touch things with robotic fingers. “It’s a tingling sensation. It’s kind of like, if your hand’s been asleep, right at the end, right before it wakes up … for me, it’s pleasant, it’s a pleasant tingling.”

In 2023, another team published research investigating a similar approach with lower leg prosthetics. They added sensors to a prosthetic foot so it could detect pressure while walking. Three volunteers with lower leg amputation went through a procedure to have electrodes implanted into the nerves of the remaining leg muscles. As the sensors in the prosthetic foot detected pressure, these implanted electrodes transmitted signals into the nerves.
For this study, the researchers first studied how nerves get stimulated naturally in response to touch, then designed their system to transmit signals in a lifelike manner. They compared this type of stimulation with constant, unchanging stimulation. When volunteers experienced the more lifelike stimulation, they walked up and down stairs faster. They said they felt more confident with this type of stimulation. The constant stimulation, in contrast, didn’t feel great. “I felt like my leg was plugged into the electricity. ” one volunteer said in a report.
Brain Implants
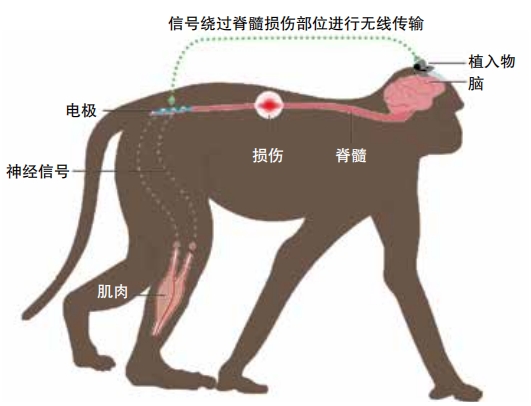
All of these examples of mind-controlled prosthetics mainly help people who lack one or more limbs. What about people who have limbs that no longer work correctly? Paralysis is a condition in which the muscles and nerves in one or more body parts do not communicate correctly with the brain. For people with paralysis, hijacking nerves in muscles or in the spine typically won’t work. “The brains of people who are paralyzed can still generate the signals that would normally control movement, but the signal can’t make it past the injury,” said John Downey, a scientist at the University of Chicago who works on neuroprosthetics. A device that sits on or inside the brain, though, can communicate signals directly to and from brain regions responsible for movement or touch even in someone with paralysis.
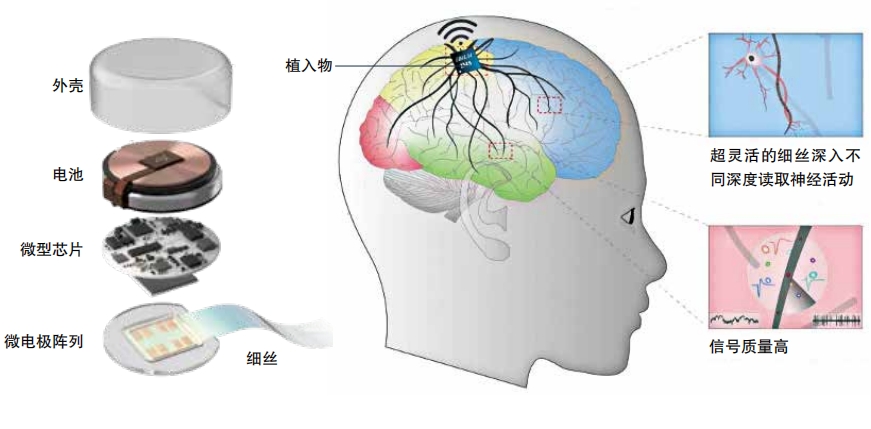
Perhaps the most famous example of a brain implant is the one Elon Musk’s company NeuraLink is working on. It is a chip with 1,000 electrodes distributed across 64 threads. A surgeon places these into the brain’s motor cortex, the region responsible for movement. After the surgery, the electrodes pick up brain activity, a computer interprets what it means, and this becomes instructions for a computer or other device.
In 2024, the company implanted human volunteers with this chip for the first time. Noland Arbaugh was the first volunteer. A swimming accident had left him quadriplegic, meaning he can’t move his arms or legs. He used his chip to play video games and control a computer. On the Lex Fridman podcast, he said, “Just having the freedom to do things on my own, at any hour of the day or night, it means the world to me.”
In the future, a chip like this could be used to control robotic arms or legs or even perhaps to reestablish communication with paralyzed limbs, allowing them to move again. In 2024 Musk wrote on X, “Long-term, it is possible to shunt the signals from the brain motor cortex past the damaged part of the spine to enable people to walk again and use their arms normally. ” There’s a very long way to go before this can be achieved, though.
NeuraLink’s volunteers weren’t the first to receive brain implants that allow control over computers or robotic limbs.Other researchers have been working toward this goal for many decades, beginning with experiments on monkeys and other animals. In 2007, the University of Pittsburgh established its Human Neural Prosthetics program. This group has worked with many volunteers who freely decide to go through brain surgery in order to help advance science.

One of those volunteers, Jan Scheuermann, had become a quadriplegic as the result of a rare disease. She received two brain implants in 2012. Tiny wires carried signals from these implants to twin posts that protruded from the top of her head. When researchers wired these posts to a computer system, they were able to read brain activity from her motor cortex. They used that activity as instructions for a robotic arm, which Scheuermann nicknamed Hector. It took some practice, but Scheuermann learned to use Hector to pick up objects. One memorable day, she lifted a chocolate bar and took a bite. “It was the best chocolate ever,” she recalled.
Brain implants have many drawbacks. Brain surgery is invasive and can have dangerous side effects such as infection. Also, these implants often stop working after several months or years. That’s because the immune system sees the implants as foreign intruders and walls them off with scar tissue. Many of the threads from Arbaugh’s implant stopped working soon after surgery, leaving him with many fewer working channels of communication than expected.
All of the impressive devices described in this story cost a lot of money, and many of them are research prototypes that can’t be used at home yet. Even once they are approved for home use, the people who need them may not be able to afford or access them. In the near future, it’s important for society to find ways to make neuroprosthetics more affordable and more widely available. Mind- controlled devices could make the world more accessible to people with a variety of disabilities. Herr is still dreaming of a world without mobility disability. “We don’t just want to build fancier and fancier robotic tools or devices,” he told CNN in 2024. “We want to rebuild human bodies. ”