Aging is a fact of life. Even if you avoid bad accidents, injuries, and infections, you won’t stay healthy forever. Your body will break down as you get older. You’ll get wrinkles and achy joints. Your vision and hearing will fade. Your chance of developing cancer, heart disease, and many other ailments will greatly increase. Aging “bedevils us all,” writes Steven N. Austad in his book, Methuselah’s Zoo. He defines aging as “the progressive deterioration over time of bodily functions and defenses along with increasing susceptibility to diseases.”

This deterioration happens because living cells tend to break down with age. Cells are the basic building block of all animals, plants, fungi, and many single-celled organisms. When cells age, the creature they belong to ages as well.

What if we could slow or stop this process? Austad and other scientists who study aging hope to find a way to treat aging just as we treat diseases. These scientists often use animals as study subjects.
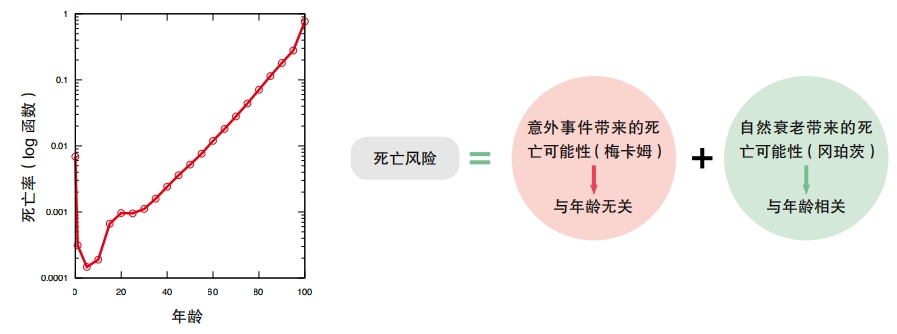
All mammals that survive into adulthood will age and die. Once aging starts, an animal’s risk of dying increases exponentially over time. We can use math to measure such a risk. The Gompertz-Makeham law of mortality is a mathematical formula that determines how risk of death changes over time. The Makeham part of the equation covers risk from external causes such as accidents. However, when an animal is well-protected in a laboratory or other safe environment, its risk of death still increases because of the internal aging processes. This is the Gompertz part of the equation.
Benjamin Gompertz first developed the law in 1825 based on records of human lifespans in Europe. His goal was to help life insurance companies more accurately calculate a person’s risk of death. Soon after, William Makeham modified the equation. Then, in the 1900s scientists began using it to model growth and survival in many types of animals and even plants and bacteria. In people and some other animals, the risk of death is lowest at sexual maturity. After that risk of death increases exponentially over time.


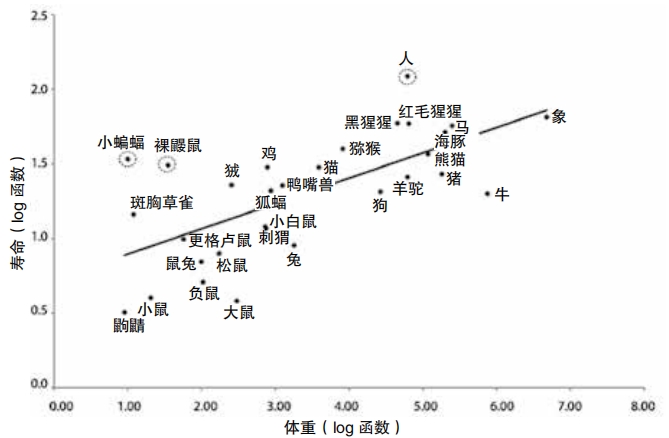
This exponential increase happens at different rates for different animals. In general, smaller animals tend to age more rapidly than larger ones. A pet mouse will live for about two years, while an African elephant may live to sixty or seventy. This is not a hard and fast rule. Opossums are about the same size as cats, yet pet cats regularly live to age twenty while opossums in captivity typically age and die before age four. Meanwhile, there are animals of small size, such as some jellyfish, lobsters, and a small freshwater creature called a hydra which do not seem to age at all.
The most common subjects of aging studies are small creatures that live short lives and age rapidly, such as mice, fruit flies, and nematodes, also called roundworms. Studying these creatures can be very helpful because researchers don’t have to wait a long time for them to get old. They can test out ideas to slow aging and see the effects quickly. “We were discovering many ways to slow the aging process in these species,” says Austad. Still, he wondered if longer-lived animals might also have something important to teach scientists about aging. Perhaps exceptionally long-lived creatures can teach us how to keep ourselves healthier for longer.


Here are four creatures that are helping to reveal secrets to a longer, healthier life.
Naked Mole-rats
If you ranked all mammals in order from cutest to ugliest, naked mole-rats would almost certainly come towards the ugly end of the list. If you ranked mammals in order by longest lifespan for their size, though, these creatures would come out near the very top.

This creature is neither a mole nor a rat. Imagine a mouse, but take away all the hair, leaving a pink, wrinkly creature with tiny eyes and huge buck teeth. Much like ants and termites, naked mole-rats live in underground colonies. A queen and a few kings produce all the pups, while the rest of the group have no babies. They specialize as workers, caretakers, or soldiers.
Though similar in size to a mouse, a naked mole-rat can live for an incredibly long time in captivity. Rochelle Buffenstein of the biotech company Calico in San Francisco, California started studying the animals over thirty years ago. As of 2022, her oldest study subject was thirty-nine years old.
Naked mole-rats show some signs of aging after twenty years. However, they stay remarkably healthy. Buffenstein reported in 2018 that their risk of dying was not increasing exponentially over time like it was supposed to. It was staying the same, year after year. “To me this is the most exciting data I’ve ever gotten,”Buffenstein told Science. “It goes against everything we know in terms of mammalian biology.”
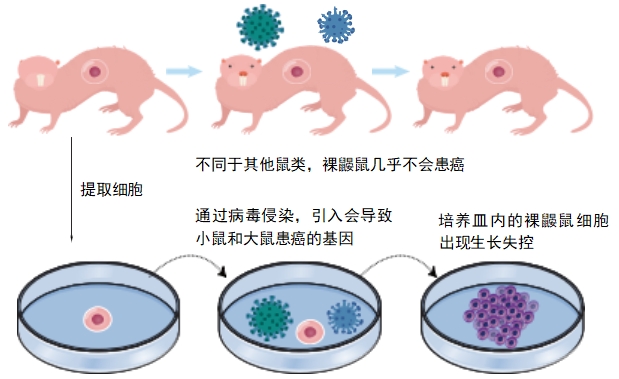
One thing that helps naked mole-rats stay healthy so long is the fact that they almost never get cancer. In a healthy body, cells divide and make more of themselves only when the body needs to grow or gets hurt and needs repairs. If cells divide out of control, that’s called cancer. Scientists used to think that something in naked mole-rat cells prevented them from growing out of control.


But in a 2020 study, researchers extracted out some naked mole-rat cells and put them into petri dishes. They then injected cancer-causing genes from mice and rats into these cells. And in the end they observed that the modified naked mole rat cells grew out of control. That means naked mole- rat cells do not have any intrinsic ability to stop themselves from growing out of control. It is something outside of the cells — something in the naked mole rat’s immune system — protects the animal from cancer. Lead study author Dr. Walid Khaled of the University of Cambridge said, “If we can understand what’s special about these animals’ immune systems and how they protect them from cancer, we may be able to develop interventions to prevent the disease in people.”

Another biological process linked to aging is oxidative stress. When a molecule with an unpaired electron (such a molecule is called a “free radical”, and often contains oxygen) interacts with other molecules in a cell, an oxidation reaction will occur, damaging the cell. Usually, there is a chemical compound found in bodies, called “antioxdiants”, which helps prevent this damage. Oxidative stress happens when the number of free radicals far exceeds the amount antioxdiants can deal with. It can cause damage to the DNA which builds up over time. This leads to symptoms of aging.
Naked mole-rats experience high levels of oxidative stress. Yet they do not have more antioxidant defenses than their short- lived mouse cousins. Scientists are still investigating why this is, but they’ve found that naked mole-rat bodies are very good at repairing DNA damage.
Naked mole-rats evolved to live in a very hazardous environment. In their burrows, they make do with very little oxygen to breathe, no light, and low amounts of food. As they adapted to deal with these stressors, their bodies likely became very good at handling the stress associated with aging as well.
Mouse-eared Bats

Gigantic bowhead whales are the world’s longest lived mammals, but if you adjust for size, that award goes to a bat. Brandt’s bat, a type of mouse-eared bat native to Northern Europe and Asia, weighs about the same as a pencil, yet can live for forty-one years in the wild. “Think about that,” writes Austad. “A bat small enough to be mistaken for a large butterfly in flight manages to avoid predators, survive famine, flood, pestilence, heat waves, and cold snaps decade after decade.” And these bats don’t just survive a long time — they remain strong, agile, and healthy as they age. An old bat must still fly for many kilometers every night while chasing the insects it eats. Plus, bats rarely get cancer and also typically harbor many viruses without developing infections.
A 2018 study revealed one reason why mouse-eared bats avoid many of the ravages of aging. The answer has to do with a special part of the DNA called a telomere. Each chromosome of DNA is shaped a bit like the letter X. Telomeres cap off all four ends of the X, acting a bit like the plastic bit at the end of a shoelace. Just as that plastic bit stops a shoelace from falling apart, a telomere protects the chromosome from getting frayed or tangled. Each time a cell divides, the telomeres of its chromosomes shorten. When they become too short, the cell stops working properly, becomes inactive, or dies. People and animals with shorter telomeres are more likely to get sick or die than those with longer ones.
Scientists think telomere shortening happens because it helps to prevent cancer. It’s like a built-in ticking time bomb. Cells can’t divide out of control if they die as their telomeres shorten. Cancer cells get around this process by producing an enzyme called telomerase that prevents telomeres from shortening.

In many bat species, telomeres naturally shorten over time just as they do in humans. But in the mouse-eared bat, this doesn’t happen, researchers reported in a 2018 study. “In the longest-lived species of bats (Myotis), we did not detect any evidence that their telomeres shorten with age, contrary to all expectations,” said study author Nicole Foley of University College Dublin, Ireland.
Some other long-lived creatures also manage to maintain telomere length over time. Some types of naked mole-rat have telomeres that shorten with age, while others maintain the length of their telomeres. Stranger yet, researchers have found evidence of telomeres that lengthen slightly in naked mole-rat blood cells. Lobsters are another example. These sea creatures produce lots of telomerase, so their cells can keep dividing and growing indefinitely. In fact, lobsters continue to grow larger and larger the longer they live. Some people have proposed using telomerase to try to prevent aging – but that’s a very risky proposition. Extra telomerase would likely increase the risk of cancer.

Mouse-eared bats, however, do not use telomerase to protect their telomeres. The researchers found none of this enzyme in their study subjects. Lead study author EmmaTeeling says that the bats likely evolved a “unique process” that allows them to maintain their telomeres without getting cancer. Her team found two genes that seem to be involved. They are called ATM and SETX. “It looks like these two genes have potentially evolved differently in bats than other mammals,” Teeling told Vice. “This is a hypothesis. This is what we need to look at next.” If her hypothesis turns out to be true, then future treatments for aging in humans could target these genes.
Red Sea Urchins
A sea urchin is a round, spiny sea creature. It has no heart, no brain, and its mouth is located underneath its body. The red sea urchin, native to shallow seas off the coast of Japan, Alaska, and California, is one of the longest-lived animals on Earth. Researchers have estimated that some live to two hundred years or more, though one hundred years is more typical. And it seems as if they do not age at all during their long lives. “No animal lives forever, but these red sea urchins appear to be practically immortal,” said Thomas Ebert of Oregon State University. “They can die from attacks by predators, specific diseases or being harvested by fishermen. But even then they show very few signs of age. The evidence suggests that a 100-year-old red sea urchin is just as apt to live another year, or reproduce, as a 10-year-old red sea urchin.”


One secret to their long lives is their ability to regenerate body parts. Human bodies can regenerate tissue in the skin, gut, and bones, but the liver is the only large, complex part that will grow back if damaged. If one of an urchin’s many tube- shaped feet or spines gets damaged, though, it rapidly grows a new one. And this ability does not decline with age. Though urchins seem completely alien when compared with mammals, they are actually more closely related to us than insects, worms, or squids. We share many genes with them. So learning how they manage their feats of regeneration and longevity could help humans live healthier lives. Other animals with remarkable regenerative abilities include jellyfish, axolotls, starfish, and sea cucumbers.
Regeneration isn’t the only way urchins break the rules of typical animal aging. In a 2020 study, researchers discovered intriguing patterns of gene expression that seem to help a red sea urchin’s nerves stay young. (An urchin has no brain, but it does have nerve tissue, which plays a similar role).The team found that the expression of 3,370 genes had changed significantly in older urchins. These changes seemed to be helping to keep its nerve tissue healthy. An urchin’s immune system also remains strong and may even strengthen with age.
Tardigrades
Tardigrades maybe the most alien species that lives on Earth. Affectionately called “water bears”, these microscopic critters are about the size of the period at the end of a sentence (They are usually less than one millimeter in length). There are many different species of water bears, and they have their own phylum, Tardigrada. They aren’t closely related to any other animal, not even insects. They thrive in watery environments, and typically only live for a few years. Yet they are considered the toughest creature on Earth, able to survive conditions that would kill any other living thing. This is what makes them interesting to people researching aging.

When conditions turn bad, a tardigrade curls up into a ball called a tun. Its body dries out and almost completely shuts down. It’s barely alive. In this state, some tardigrade species can survive for thirty years with no food or water. Like a real-life Sleeping Beauty, the tardigrade doesn’t seem to age while in a tun. The tun state also helps this remarkable animal to resist extreme cold and even survive the vacuum and intense radiation of outer space. Astronauts put a bunch of tardigrades on the outside of a space capsule for twelve days, and they revived easily when they were returned to ideal conditions.
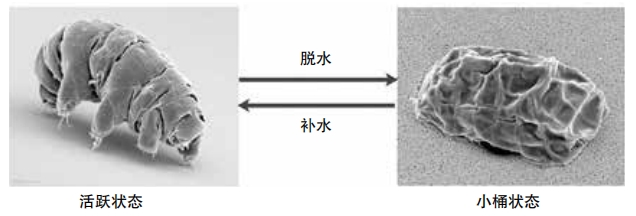
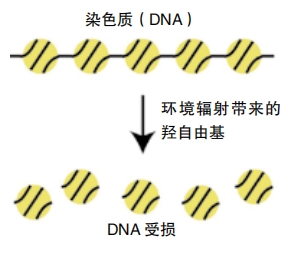
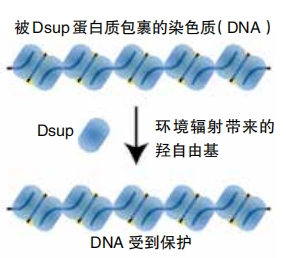
The fact that tardigrades survive radiation damage is especially interesting to scientists and researchers studying aging. When people are exposed to unsafe levels of radiation, their DNA gets damaged, leading to an increased risk of cancer and other diseases. A 2016 study in Nature Communications found that tardigrades produce a special protein that coats and protects their DNA from such damage. The researchers named it Dsup, short for “damage suppressor.”
Ingemar Jönsson studies tardigrades at Kristianstad University in Sweden and wasn’t involved in the 2016 study. He told Nature, “Protection and repair of DNAis a fundamental component of all cells and a central aspect in many human diseases, including cancer and aging.” Scientists may someday find a way to use Dsup to protect human DNA, making our cells more resilient. Researchers are looking for similar proteins that may protect tardigrades when they lack oxygen or water. Perhaps these types of proteins could help protect humans, too.
The Future Human Healthspan
For our size, humans actually live much longer than most mammals. And thanks to modern technology and medicine, we’ve made it possible to avoid many of the medical and environmental perils that used to cut our lives short. Sadly, aging has remained inevitable. More and more people live past the age of eighty every year, yet they all become old and frail. If this trend continues, writes Austad, “More and more people, living longer and pushing up against a limit of human life, could require more and more medical help and could live more and more years in pain — demented and disabled.”
Many experts think we can avoid this future, but only if we learn how to treat aging. Austad thinks the treatments we need are already out there in the animal kingdom, hidden among the long-lived creatures that show incredible resilience in the face of difficult and demanding conditions.
