Genetic engineering has produced some amazing species. One type of corn has a gene from bacteria. The corn is safe for people to eat, but the plant poisons caterpillars that try to attack it.There’s also a salmon that grows to market weight in less than half the usual time of three years. It’s an Atlantic salmon with genes from a Pacific chinook salmon.
Horizontal gene transfer, or HGT, makes these and dozens of other bio-engineered species possible. Usually in nature, genes get passed down vertically, from parent to offspring. Genetic engineering with HGT takes genes from one or more species and adds them to a different species. The goal is to add desirable traits to improve farming, produce medicines, and more.
Doing that presents lots of challenges. Having everything work right takes skill and lots of luck.
Perhaps even more amazing is that horizontal gene transfer sometimes happens in nature—without any science labs. In 1928, Frederick Griffith, a British scientist, used R and S Bacteria strains to experiment with mice, and concluded that something from the dead S bacteria must have transformed the non-harmful R bacteria.
In 1944, scientists at the Rockefeller Institute in New York explained that the dead S bacteria must have released their DNA, and some of the live R bacteria were able to add the DNA bits that coded for the protective cell coating. This happens because different types of restriction enzymes can cut DNA molecules apart. Other enzymes, called ligases, let fragments of DNA attach themselves to cut-apart spots having the right sequences of codes. Other types of bacteria also can pick up genes from their environment in this way.
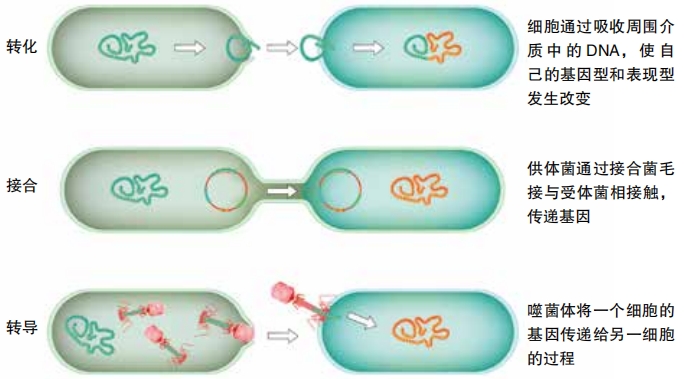
Bacteria also can transfer genes to each other by conjugation. In that process, certain bacterial cells use a structure called a pilus to pull another cell close and attach to it. The donor transfers a copy of a DNA ring, called a plasmid, to the other cell. Then the cells separate.
Bacteria also can get genes from viruses called bacteriophages. The virus infects a cell, turning it into a factory to make more of the virus. Sometimes, though, bits of the virus DNA get taken up by the cell’s own DNA. The cell passes the altered genome along to offspring.
However it happens, the added genes can be harmful or helpful. Even if the genes help the bacteria, they can be bad for people and other species. In Griffith’s work, the added genes made the bacteria harmful to mice. And sometimes, transferred genes help bacteria resist antibiotic medicines, helping the bacteria to remain alive and continue to be harmful to other species.
Beyond Bacteria
Horizontal gene transfer happens less often in species whose cells have distinct nuclei. Yet studies within the last twenty years show it’s happened thousands of times.
“Such transfers can occur between two closely related species, such as between two species of flies,” says Clément Gilbert of the University of Paris-Saclay in France. Transfers also can happen “between very distantly related species, such as a bug and a monkey.” Those species’ last common ancestor lived more than 500 million years ago. “A good understanding of horizontal transfer may allow us to reconstruct past interactions between species,” Gilbert says. In one 2017 paper, he and a colleague explained that transfer can take place from virus to host. Or a host can transfer genes to a virus.
Researchers in Britain and Canada reported roughly twice as many host-to-virus transfers as virus-to-host cases in their 2021 study, Various enzymes produced by viruses and host cells appear to promote the process. Stress on the host cell and the cell processes for breaking down proteins also appear to play roles.
When species’ lives intertwine with each other, there are more opportunities for transfer. Plants living very close to each other or even grafted together might transfer genes through membrane channels that can cross cell walls. Parasites or symbiotic organisms might also transfer genes through such channels. Fungi also have gotten genes through HGT.
Animals likewise have gotten DNA from other species. Shared metabolic pathways between animals and symbiotic microbes are one way this might happen, a 2014 paper suggested. Symbiotic or parasitic organisms might also act as vectors for transferring DNA between species.
Scientists know HGT has happened in animals thanks to computer analysis of published genomes for many species. They look for cases where bits of DNA were shared with species that were not closely related in an evolutionary sense.
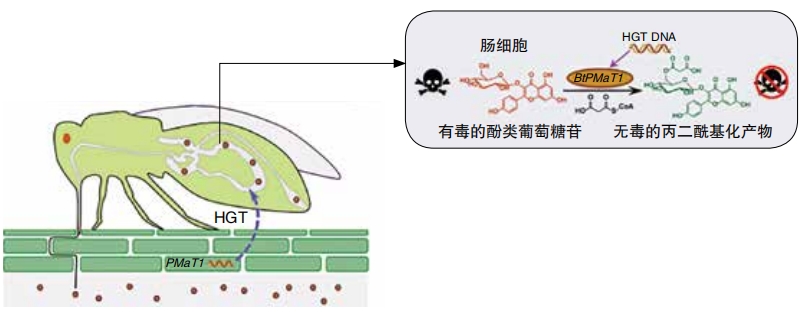
One 2021 study focused on the sweet potato whitefly. The insect can destroy many types of crops. Some plants produce poisons to defend themselves. But the whitefly has a gene that lets it neutralize the poisons. When it conducted the 2021 study, the team knew what the gene was, but the team didn’t find the gene in other insects. Instead, they found the gene in plants.
“Insects have obviously not evolved from plants,” explains Ted Turling at the University of Neuchâtel in Switzerland, who worked on the study. So, “the fact the gene is not in any other insect implies that it was horizontally transferred.” People hadn’t known about plant-to-animal gene transfers before. “Being the first to show this is very exciting,” Turling says. He hopes the knowledge will help scientists find new ways to control the pest.
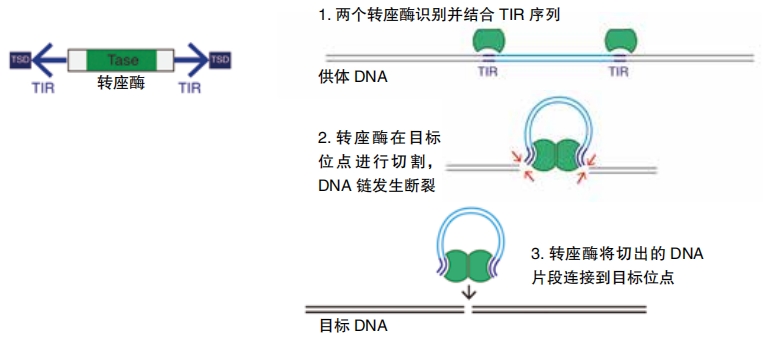
In another study, Gilbert and other researchers screened the genomes of 307 animals with backbones, called vertebrates. The team focused on bits of DNA called transposable elements, or TEs. TEs are also known as jumping genes. As they copy themselves, they can change position on an organism’s chromosomes. Basically, enzymes within the cell make it easy for these DNA sequences to be cut and pasted into other positions on the genome. This quality also makes them more likely to be cut and pasted into the genomes of other organisms. So, they are likely candidates for becoming added DNA.
Gilbert’s group found at least 975 genes that appear to have come from other species that were not closely related in an evolutionary sense. More than 90 percent of the cases involved fishes. Less than 3 percent were mammals. In other work, Gilbert and others found more than two thousand instances of transferred TE’s among 195 insect genomes.
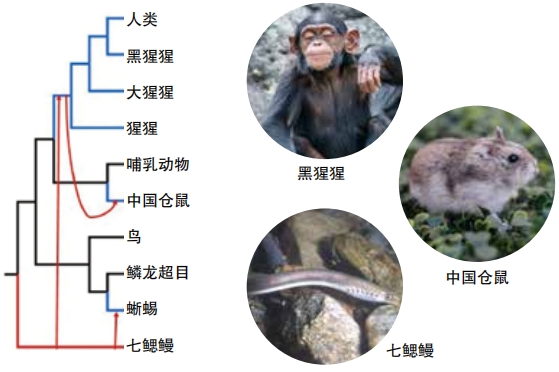
Studies show humans have likely gotten genes from other species as well. Chaochun Wei is at Shanghai Jiao Tong University in China. In one study in 2017, he and other researchers compared large parts of the human genome with those of other mammals and some non-mammal vertebrates. The group looked for human DNA sequences longer than one thousand base pairs that also showed up in at least two non- mammals, but that didn’t show up in more than a few other mammals. The idea was to rule out genes that probably were passed along from parent to offspring in the normal course of evolution.
Later work by Wei’s group has identified about three hundred cases where the human genome likely got DNA through horizontal gene transfers.That’s fewer than the group originally reported. “But it is still big enough to claim the existence of HGTs in humans,” Wei says. Parasites that spend part of their lives in non-mammals and part in mammals might have transferred the genes, his group’s 2017 paper suggested.
Knowing that HGT has happened in humans and other animals as well as in plants, fungi, bacteria, and other organisms changes our understanding about evolution. “If horizontal gene transfer has taken or can take place with humans or other animals, we have a web of life, not a tree of life,” Wei says.
Genetic Engineering with HGT
HGT in nature happens by chance, but scientists in labs are very deliberate about their work. First, they figure out the DNA code for genes to provide traits they want another species to acquire. They make many copies of that DNA sequence.
The scientists then package that DNA with other genes. Often, scientists include parts from some viral DNA. The scientists remove parts of a virus that could harm a target organism. But they keep parts of the virus DNA that let it insert itself—and the rest of the gene package—into a cell’s DNA.
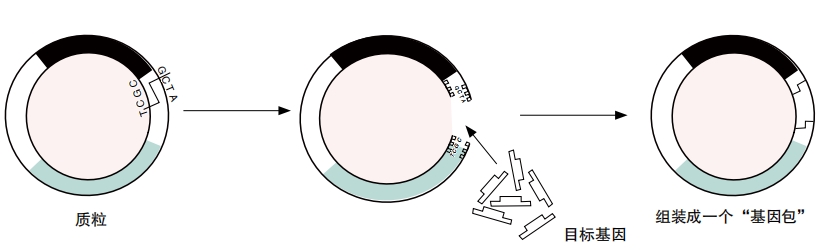
The package may also have genes that act as a promoter. A promoter makes it more likely that the genes in a package will be expressed, or turned on. If a gene is expressed, then the organism should exhibit the trait it provides.
Even with all of that, a gene package might get incorporated into the genome of only a few among thousands of cells in a lab. Scientists want a way to know which cells those are. So they usually add a marker gene to the package. Green fluorescent protein, or GFP, is a common marker. It makes cells glow under ultraviolet lighting.
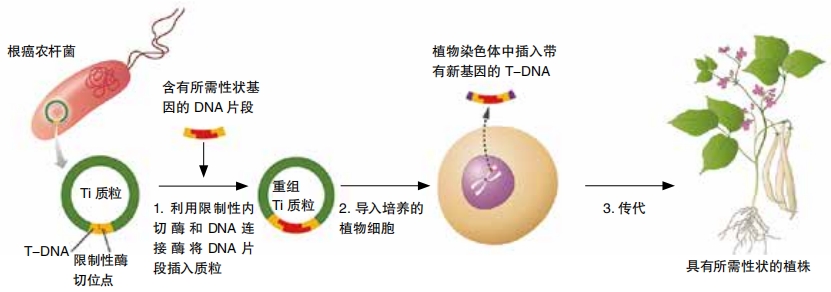
Researchers use different ways to get the gene packages into cells. The method depends on the target species. Packages arranged as plasmids can be mixed with a culture of bacteria, for example. For other organisms, a lab might use electric fields or low-frequency ultrasound to destabilize the target cells’ membranes. Then the gene package can get in. The genetic material might also be coated onto little bits of gold or tungsten. Then a gene gun can shoot the bits into cells.
Work doesn’tend when the gene package gets in. Scientists must make sure the new organisms stay healthy as they grow, the species expresses the trait, and any crop or medicine affected is safe for people. All of this takes time.

Most engineered species developed with HGT have been plants for agriculture. For example, Roundup Ready corn and soybeans have a gene that protects them from the poison in a weed killer sold as Roundup. Farmers can plant their crops and then spray the weed killer. Weeds die, but the crops live. HGT also can help make medicines. For example, genetically engineered bacteria can produce human insulin.
There is often controversy, too. Some environmental groups worry about whether herbicide-resistant crops are really safe if farmers have sprayed the plants with lots of weed killer. Other groups worry about possible cruelty to animals developed with horizontal gene transfer.
Meanwhile, scientists continue to work on genetic engineering. And they continue to study HGT’s role in nature and evolution.
“A lot of surprising results are waiting for us to find,” Wei says.